Carolina Naturally
Carolina Naturally is proud to bring you an excerpt from the book
NOTHING: Surprising Insights Everywhere from Zero to Oblivion, from the magazine
New Scientist.
It features thoughts from 21 different writers and scientists on
subjects from the nature of nothingness to the cosmos to the inner
workings of the human mind. They harness the latest research to explain
complicated concepts in ways we can understand. For example:
Why does the placebo effect—essentially, feeling better after taking nothing—work?
Can meditation—clearing the mind of everything—cause structural changes in the brain?
What was happening immediately after the big bang, and, even more mysterious, what came before?
Is the appendix—a body part that supposedly does nothing—truly a vestigial organ?
Why was it so hard to invent the number zero?
How might cooling elements down to nearly absolute zero solve our energy crisis?
This excerpt on the big bang is from
New Scientist cosmology consultant
Marcus Chown.
Beginnings
“Astronomy
leads us to a unique event, a universe which was created out of
nothing,” said Arno Penzias, the American physicist and Nobel laureate.
He was talking about the mother of all beginnings, the big bang. It’s
the obvious place for us to start. To add some variety, we’ll bounce you
to ancient Babylon and then to the most modern of brain-scanning
laboratories. You’ll find out about the birth of a symbol that you
almost certainly take for granted and discover that your head is home to
an organ you’ve probably never heard of. Along the way, we’ll look at
the fruits of an infant scientific field—the mind’s power to heal the
body.
The big bang
Our universe began in
an explosion of sorts, what’s called the big bang. The $64,000 question
is how the cosmos emerged out of nothing. But before we tackle that, we
need to understand what the big bang entailed. Here’s Marcus Chown.
In
the beginning was nothing. Then the universe was born in a searing hot
fireball called the big bang. But what was the big bang? Where did it
happen? And how have astronomers come to believe such a ridiculous
thing?
About 13.82 billion years ago, the universe that we
inhabit erupted, literally, out of nothing. It exploded in a titanic
fireball called the big bang. Everything—all matter, energy, even space
and time—came into being at that instant.
In the earliest moments
of the big bang, the stuff of the universe occupied an extraordinarily
small volume and was unimaginably hot. It was a seething cauldron of
electromagnetic radiation mixed with microscopic particles of matter
unlike any found in today’s universe. As the fireball expanded, it
cooled, and more and more structure began to “freeze out.”
Step
by step, the fundamental particles we know today, the building blocks of
all ordinary matter, acquired their present identities. The particles
condensed into atoms and galaxies began to grow, then fragment into
stars such as our sun. About 4.55 billion years ago, Earth formed. The
rest, as they say, is history.

It
is an extraordinarily grand picture of creation. Yet astronomers and
physicists, armed with a growing mass of evidence to back their
theories, are so confident of the scenario that they believe they can
work out the detailed conditions in the early universe as it evolved,
instant by instant.
It is an extraordinarily grand picture of creation. Yet astronomers
and physicists, armed with a growing mass of evidence to back their
theories, are so confident of the scenario that they believe they can
work out the detailed conditions in the early universe as it evolved,
instant by instant.
That’s not to say we can
go back to the moment of creation. The best that physics can do is to
attempt to describe what was happening when the universe was already
about 10–35 seconds old—a length of time that can also be written as a
decimal point followed by 34 zeroes and a 1.
This is an
exceedingly small interval of time, but you would be wrong if you
thought it was so close to the moment of creation as to make no
difference. Although the structure of the universe no longer changes
much in even a million years, when the universe was young, things
changed much more rapidly.
For example, physicists think that as
many important events happened between the end of the first tenth of a
second and the end of the first second as in the interval from the first
hundredth of a second to the first tenth of a second, and so on,
logarithmically, back to the very beginning. As they run the history of
the universe backward, like a movie in reverse, space is filled with
ever more frenzied activity.
This is because the early universe
was dominated by electromagnetic radiation—in the form of little packets
of energy called photons—and the higher the temperature, the more
energetic the photons. Now, high-energy photons can change into
particles of matter because one form of energy can be converted into
another, and, as Einstein revealed, mass (m) is simply a form of energy
(E), hence his famous equation E=mc2, where c is the speed of light.
What
Einstein’s equation says is that particles of a particular mass, m, can
be created if the packets of radiation, the photons, have an energy of
at least mc2. Put another way, there is a temperature above which the
photons are energetic enough to produce a particle of mass, m, and below
which they cannot create that particle.

If
we look far enough back, we come to a time when the temperature was so
high, and the photons so energetic, that colliding photons could produce
particles out of radiant energy. What those particles were before the
universe was 10–35 seconds old, we do not know. All we can say is that
they were very much more massive than the particles we are familiar with
today, such as the electron and top quark.
As time progressed
and temperature fell, so the mix of particles in the universe changed to
a soup of less and less massive particles. Each particle was “king for a
day,” or at least for a split second. For the reverse process was also
going on—matter was being converted back to radiant energy as particles
collided to produce photons.
What do physicists think the universe was like a mere 10–35 seconds after the big bang?
Well,
the volume of space that was destined to become the “observable
universe,” which today is 84 billion light years across, was contained
in a volume roughly the size of a pea. And the temperature of this
superdense material was an unimaginable 1028 ºC.
At this
temperature, physicists predict, colliding photons had just the right
amount of energy to produce a particle called the X-boson that was a
million billion times more massive than the proton. No one has yet
observed an X-boson, because to do so we would have to recreate, in an
Earth bound laboratory, the extreme conditions that existed just 10–35
seconds after the big bang.
How far back can physicists probe in their laboratories?
The
answer is to a time when the universe was about one-trillionth (10–12)
of a second old. By then, it had cooled down to about 100 million
billion degrees—still 10 billion times hotter than the center of the
sun. In 2012, physicists at CERN, the European center for particle
physics in Geneva, recreated these conditions in the giant particle
accelerator called the Large Hadron Collider. They conjured into being a
particle that resembles the Higgs boson, a particle that vanished from
the universe a trillionth of a second after the big bang.

The
gulf between 10–35 seconds and a trillionth of a second is gigantic. We
know that for most of this period, matter was squeezed together more
tightly than the most compressed matter we know of—that inside the
nuclei of atoms. And, as the temperature fell, so the energy level of
photons declined, creating particles of lower and lower masses.
At
some point, the hypothetical building blocks of the neutron and
proton—known as quarks—came into being. And by the time the universe was
about one-hundredth of a second old, it had cooled sufficiently to be
dominated by particles that are familiar to us today: photons,
electrons, positrons and neutrinos. Neutrons and protons were around,
but there weren’t many of them. In fact, they were a very small
contaminant in the universe.
About one second into the life of
the universe, the temperature had fallen to about 10 billion ºC, and
photons had too little energy to produce particles easily. Electrons and
their positively charged “antimatter” opposites, called positrons, were
colliding and annihilating each other to create photons. However,
because of a slight and, to this day, mysterious lopsidedness in the
laws of physics, there were roughly 10 billion + 1 electrons for every
10 billion positrons. So, after an orgy of annihilation, the universe
was left with a surplus of matter, and with about 10 billion photons for
every electron, a ratio that persists today.
The next important stage in the history of the universe was at about one minute.
The
temperature had dropped to a mere 1 billion ºC—the temperature in the
hearts of the hottest stars. Now the particles were moving more slowly.
In the case of protons and neutrons, it meant that they stayed close to
each other long enough for the strong nuclear forces, which bind them
together in the nuclei of atoms, to have a chance to take hold. In
particular, two protons and two neutrons could combine to form nuclei of
helium.
Solitary neutrons decay into protons in about 15
minutes, so any neutrons left over after helium formed became protons.
According to physicists’ calculations, roughly ten protons were left
over for every helium nucleus that formed. And these became the nuclei
of hydrogen atoms, which consist of a single proton.

This is one
of the strongest pieces of evidence that the big bang really did happen.
For much, much later, when the temperature had cooled considerably, the
hydrogen and helium nuclei picked up electrons to become stable atoms.
Today, when astronomers measure the abundance of elements in the
universe—in stars, galaxies and interstellar space—they still find
roughly one helium atom for every ten hydrogens.
The
point at which it was cool enough for electrons to combine with protons
to make the first atoms was about 380,000 years after the big bang. The
universe was now cooling very much more slowly than in its early
moments, and the temperature had reached a modest 3,000 ºC. This also
marked another significant event in the early history of the universe.
Until
the electrons had combined with the hydrogen and helium nuclei, photons
could not travel far in a straight line without running into an
electron. Free electrons are very good at scattering, or redirecting,
photons. As a consequence, every photon had to zigzag its way across the
universe. This had the effect of making the universe opaque. If this
happened today and light from the stars zigzagged its way across space
to your eyes, rather than flying in straight lines, you would see only a
dim milky glow from the whole sky rather than myriad stars.
We
can still detect photons from this period. They have been flying freely
through the universe for billions of years, and astronomers observe them
as what’s called the cosmic microwave background. Whereas these photons
started their journey when the temperature was 3,000 ºC, the universe
has expanded about 1100 times while they have been in flight. This has
decreased their energy by this factor, so that we now record the signals
as just 2.725 degrees above absolute zero.
The temperature
dropping to about 3,000 ºC also signalled another event—the point at
which the energy levels of the radiation, or photons, in the universe
fell below that of the matter. From then on, the universe was dominated
by matter and by the force of gravity acting on that matter.

The
building of elements, which had begun when the universe was about one
minute old, had stopped by the time it had been in existence for ten
minutes, and the protons and neutrons had formed the nuclei of hydrogen
and helium. For elements such as carbon and oxygen to form, hotter and
denser conditions were needed, but the universe was getting colder and
more rarefied all the while. The heavy elements in the planets and in
your body were created, billions of years later, in the nuclear furnaces
of stars.
Instead,
as the universe continued to expand, gravity caused clumps of matter to
accumulate in large islands. Those islands were to become the galaxies.
The galaxies continued their headlong rush into the void, fragmenting
into smaller clumps which became individual stars, producing heat and
light by nuclear reactions deep in their cores. At one point, about 9
billion years after the big bang, a yellow star was born toward the
outer edge of a great spiral whirlpool of stars called the Milky Way.
The star was our sun.
How do we know there was a big bang?
Our
modern picture of the universe is due in large part to an American
astronomer, Edwin Hubble. In 1923, he showed that the Milky Way, the
great island of stars to which our sun belongs, was just one galaxy
among thousands of millions of others scattered throughout space.
Hubble
also found that the wavelength of the light from most of the galaxies
is “red shifted.” Astronomers initially interpreted this as a Doppler
effect, familiar to anyone who has noticed how the pitch of a police
siren drops as it passes by. The siren becomes deeper because the
wavelength of the sound is stretched out. Similarly with light, the
wavelength of light from a galaxy which is moving away from us is
stretched out to a longer, or redder, wavelength.
Hubble
discovered that most galaxies are receding from the Milky Way. In other
words, the universe is expanding. And the farther away a galaxy is, the
faster it is receding.

One
conclusion is inescapable: the universe must have been smaller in the
past. There must have been a moment when the universe started expanding:
the moment of its birth. By imagining the expansion running backward,
astronomers deduce that the universe came into existence about 13.82
billion years ago.
This idea of a big bang means that the red
shifts of galaxies are not really Doppler shifts. They arise because in
the time that light from distant galaxies has been traveling across
space to Earth, the universe has grown, stretching the wavelength of
light.
The picture of a universe that is expanding need not have
been a surprise to anyone. If Albert Einstein had only had faith in his
equations, he could have predicted it in 1915 with his theory of
gravity, known as the general theory of relativity. But Einstein, like
Newton before him, hung on to the idea that the universe was
static—unchanging, without beginning or end. He can be forgiven because,
at the time, he did not even know about the existence of galaxies.
The
vision of a static universe also appealed strongly to astronomers. In
1948, Hermann Bondi, Thomas Gold and Fred Hoyle proposed the
steady-state theory of the universe. The universe was expanding, they
said, but perhaps it was unchanging in time.
Their theory said
that space is expanding at a constant rate but, at the same time, matter
is created continuously throughout the universe. This matter is just
enough to compensate for the expansion and keep the density of the
universe constant. Where this matter would come from, nobody could say.
But neither could the proponents of the big bang.
The
steady-state theory held its own as the principal challenger to the big
bang theory for two decades. Then, in the 1960s, two astronomical
discoveries dealt it a fatal blow.
The first discovery came from
Martin Ryle and his colleagues at the University of Cambridge. They were
studying radio galaxies—enormously powerful sources of radio waves. In
the early 1960s, the Cambridge astronomers found that there were many
more radio galaxies at large distances than nearby.
The radio
waves from these distant objects have taken billions of years to reach
us. Ryle and his colleagues, therefore, were observing our universe as
it was in an earlier time. The excess of radio galaxies at great
distances had to mean that conditions in the remote past were different
from those today. A universe which changes with time ran counter to the
steadystate theory.
Then in 1965, Arno Penzias and Robert Wilson,
two scientists at the Bell Telephone Labs in Holmdel, New Jersey,
detected an odd signal with a radio horn they had inherited from
engineers working on Echo 1 and Telstar, the first communication
satellites.
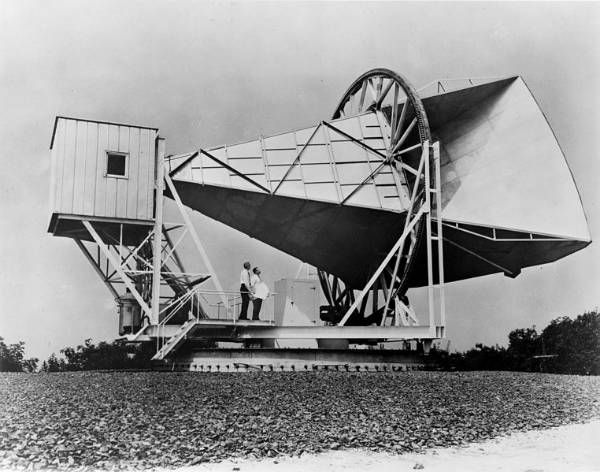
The
signal did not come from Earth or the sun. It seemed to come from all
over the sky, and it was equivalent to the energy emitted by a body at
about 3 degrees above absolute zero (–270 °C).
There could be no
doubt. Penzias and Wilson had discovered the “afterglow” of the big bang
fireball—the cosmic microwave background. For their proof of the big
bang, they shared the 1978 Nobel prize in physics.
Looking backward in time
Physicists
can run the expansion of the universe backward. In this way, they can
watch it get hotter as it gets smaller, just as the air in a bicycle
pump heats up as it is compressed. But theory proposes that, at the big
bang itself, the temperature was infinite. And infinities warn
physicists that theories are flawed.
At the moment, the theories
which take us furthest back in time are the Grand Unified Theories.
These GUTs are an attempt to show that three of the basic forces that
govern the behavior of all matter—the strong and weak nuclear forces and
the electromagnetic force—are no more than facets of a single
“superforce.”
Each force of nature arises from the exchange of a
different “messenger” particle, or boson. The messenger transmits a
force between two particles, just as a tennis ball transmits to a player
the force of an opponent’s shot. At high enough temperatures— such as
those when the universe was 10–35 seconds old—physicists believe the
electromagnetic and strong and weak nuclear forces were identical, and
mediated by a messenger dubbed the X-boson.
Physicists want to
show that gravity, too, is a facet of the superforce. They suspect that
gravity split apart from the other three forces at about 10–43 seconds
after the big bang. But before they can “unify” the four forces, they
must describe gravity using quantum theory, which is hugely successful
for describing the other forces. To say that physicists are finding this
difficult is an understatement.
When they have their unified
theory, physicists believe that they will be able to probe right back to
the moment of creation and explain how the universe popped suddenly
into existence from nothing 13.82 billion years ago.