
Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory announced today that they have observed a rare property in a special class of metals called multiferroics: they have both magnetic and electric properties, which normally don’t happen in the same material. Ferromagnets are, of course, magnetic metals, and ferroelectrics are materials that have a permanent electric polarization.
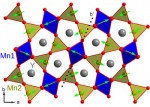
Now, scientists have found a new way that electric and magnetic properties can be coupled in a material. The group used extremely bright beams of x-rays at Brookhaven’s National Synchrotron Light Source (NSLS) to examine the electronic structure of a particular metal oxide made of yttrium, manganese, and oxygen. They determined that the magnetic-electric coupling is caused by the outer cloud of electrons surrounding the atom.
“Previously, this mechanism had only been predicted theoretically and its existence was hotly debated,” [Brookhaven physicist Stuart] Wilkins said.
In this particular material, the manganese and oxygen electrons mix atomic orbitals in a process that creates atomic bonds and keeps the material together. The researchers’ measurements show that this process is dependent upon the magnetic structure of the material, which in this case, causes the material to become ferroelectric, i.e. have an electric polarization. In other words, any change in the material’s magnetic structure will result in a change in direction of its ferroelectric state. By definition, that makes the material a multiferroic.
You’ll find more technical information at the Brookhaven National Laboratory
site.
 Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory announced today that they have observed a rare property in a special class of metals called multiferroics: they have both magnetic and electric properties, which normally don’t happen in the same material. Ferromagnets are, of course, magnetic metals, and ferroelectrics are materials that have a permanent electric polarization.
Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory announced today that they have observed a rare property in a special class of metals called multiferroics: they have both magnetic and electric properties, which normally don’t happen in the same material. Ferromagnets are, of course, magnetic metals, and ferroelectrics are materials that have a permanent electric polarization.
No comments:
Post a Comment